Below is an excerpt on technology transfer in cell therapy manufacturing originally published in the April 2015 issue of BioProcess International as part of the article, “Are You Ready for a Tech Transfer? Part 1: Challenges and Critical Factors for Success in Cell Therapy Development," written by PCT experts Catherine McIntyre, PhD, Director, Technical Operations and Cenk Sumen, PhD, Manager, Technology and Business Development.
“Cell therapies offer enormous promise for treatment of a range of conditions by replacing damaged tissue or leveraging the body’s own resources to heal itself. Not surprisingly, the cell therapy industry is growing rapidly and is poised to have a major impact on healthcare and disease treatment. The Alliance for Regenerative Medicine (ARM) has reported on the robust state of the industry, noting that revenue from cell-derived products grew from US $460 million in 2010 to $1.3 billion in 20131.
A critical aspect of cell therapy development is transfer of knowledge from a development organization to a manufacturing organization. The development organization (product sponsor) is generally the transferring site. A contract manufacturing or contract development and manufacturing organization (CMO or CDMO, respectively) often is the receiving site. In general, technology transfer to a CDMO is advised when a sponsor company requires the resources, capacity, facilities, and/or expertise directly relevant to cell therapy to meet industrial and regulatory requirements needed to advance a therapeutic product toward commercialization. So it is essential for a sponsor company to evaluate the strategy, timelines, and infrastructure necessary for a successful and streamlined technology transfer. In one sense, a rapid transition to good manufacturing practice (GMP) manufacturing at the receiving site may seem like an obvious goal. But in practice, such reckless speed can lead to an incomplete transfer or an unstable process and/or analytical methods. Consequently, that can lead to unnecessary deviations and additional time and resources required to overcome them.
Key factors in technology transfer include having a dedicated and multidisciplinary team, an attention to detail, a focus on timeline and cost, and the experience to recognize risks and mitigate them as appropriate when they appear during the transition. In some cases, the transferring site might be different from that of the originator of the process. The process may have been licensed from a hospital, an academic institution, or another company. Alternatively, the process may require transfer from one CDMO to another. The experience and knowledge of the process originator is fundamental and foundational to success and should be leveraged during a transition.
Transfer of patient-specific therapies can be complicated by the variable nature of starting raw materials. Unlike off-the-shelf processes (in which a master cell bank serves as a consistent and well-defined starting material with patient-specific therapies), starting materials can vary dramatically from patient to patient depending on severity and/or type of illness, age, and differences in cell population percentages. Furthermore, in many situations, material from healthy individuals is used for process development as well as training, engineering, and qualification runs. That material may not represent the variability seen in the affected patient population.
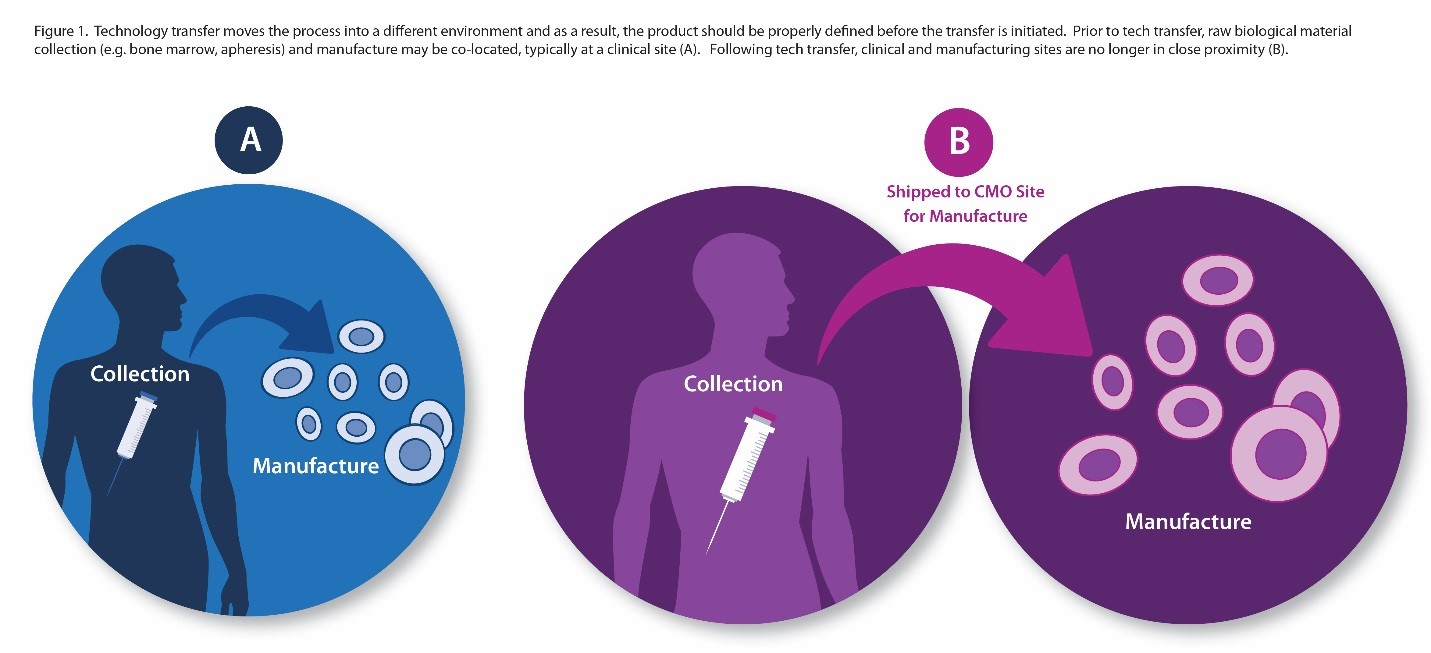
Adding to that complexity is the fact that transfer of patient-specific therapies separates the clinical site (and patients) from the manufacturing site. Consider the scenario outlined in Figure 1, in which a therapeutic product is developed at an academic medical center. Before technology transfer, collection of biological raw material through apheresis, for example, and manufacturing of the therapeutic product takes place within close physical proximity. Once the process is transferred to a CDMO, collection of starting materials and manufacture of the therapeutic product are separated by time and distance. In such cases, it is critical to establish that starting materials (e.g., apheresis product) will retain key quality characteristics during shipment and will remain stable during shipment from collection site to manufacturing site.
Partnering with an experienced CDMO allows the transferring site to identify and overcome those and other challenges and use specific services and capacity as required by project timelines and fiscal and fundraising constraints. A CDMO also can facilitate process integration and execute seamless transitions through technology transfer stage checkpoints as well as incorporate key analytical method-development goals. Application of a systematic approach during such a critical transition can help ensure timely progress toward — and achievement of — milestones.”
1 Mackay, G. State of the Regenerative Medicine Industry. Alliance for Regenerative Medicine Annual Meeting 2014, 13 January 2014; http://alliancerm.org/sites/default/files/ ARM_SOTI_PPT_2014.pdf
To read the complete article in BioProcess International, click the button below:
