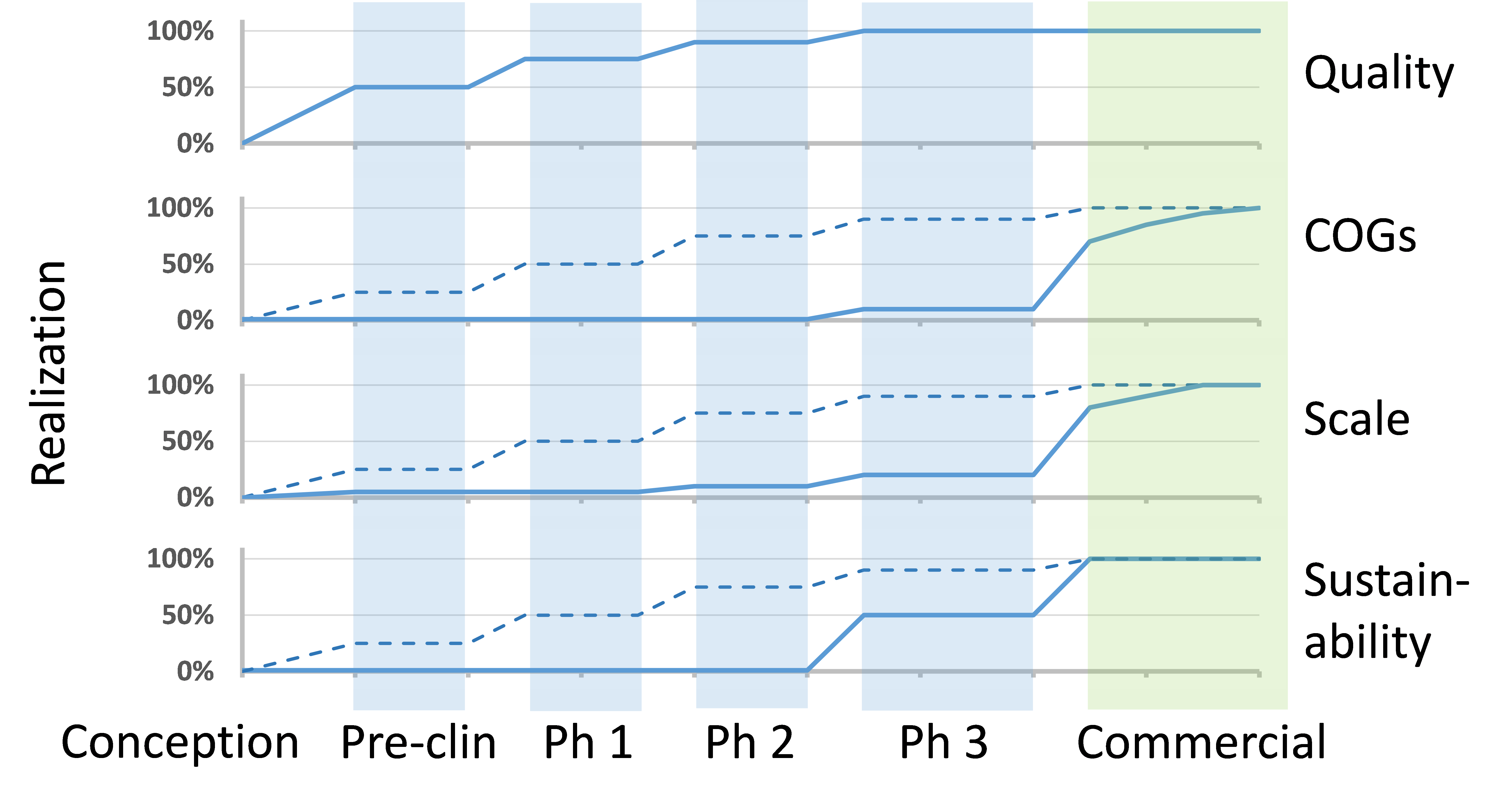
As discussed in my previous post, FDA has provided guidance via ICH Q8 for pharmaceutical development (where Quality by Design (QbD) principles are presented) for establishment of a Quality Target Product Profile (QTPP). The concept of Development by Design (DbD) takes this one step further, whereby each of the critical aspects leading to viable commercial manufacturing are addressed, including not only quality, but also cost of goods (COGs), scalability, and sustainability.
Consider each of the Development by Design attributes and their challenges:
QUALITY: Quality is certainly foundational, as recognized by QbD, but for cell therapy—where there is heavy reliance on process to meet critical quality attributes (CQAs) of the final product—the manual, open, and human-dependent nature of many process steps presents substantial risk. Given that the process is only as strong as its weakest link, then, taking the example of a patient-specific product (where there is only one patient per lot), the strength of the process is directly related to reducing the risk of failing to treat the patient. Additionally, it is often impractical to perform the complete range of lot-release tests that would be required to confirm that all CQAs have been met for each lot; therefore, a robust process with validated ability to produce products of consistently high quality is critical. Automation, integration, and closed-system design are key tactics to elevate robustness of the process.
COGS (COST OF GOODS SOLD): The current high COGs of cell therapy products (typically driven by labor and testing costs for patient-specific products and by media for off-the-shelf products) usually demands a sizable commercial value proposition. As processes mature, the focus on COGs for commercial viability becomes critical. Development by Design allows for prospective approaches to addressing COGs appropriate for scale and stage of development.
SCALABILITY: Migrating from a clinical-scale process with the capacity to make tens to hundreds of patient doses per year, on to a commercial-scale process with the capacity to make thousands to ten-thousands of patient doses, can present significant comparability risks. In particular, cell therapy products inherently possess high complexity with one or more mechanisms of action that are often incompletely understood. In addition, there is currently a lack of analytical tools and in vivo models to judge product comparability.
SUSTAINABILITY: Finally, even if quality, COGs, and scale objectives have been met, there is the very real risk that manufacturing cannot be sustained over the full product life cycle. For example, a key risk is disruption in the relatively fragile and immature supply chain currently supporting cell therapy, which could halt manufacturing for an extended period of time. In the worst case, a process step relies on supply chain elements that become no longer available and requires changes to be developed, tested, and comparability demonstrated. To mitigate risks to sustainability, companies need to assess the full range of supply chain inputs to the manufacturing process, including reagents, consumables, equipment, and human resources. Additionally, the assessment should methodically include every unit operation, both process and testing.
Considering DbD early on does not mean that a cell therapy developer needs to make a large investment much earlier on in the process, but it does mean they need to be planning ahead. Certainly, quality is a key focus from the start, but working through all of these areas mentioned at an early stage can provide significant cost and time advantages as a cell therapy developer moves further along the clinical process.

Development by Design: Initiate Early
To learn more about Development by Design, click the button below to request a complementary copy of the white paper, “Manufacturing Cell Therapies: Development Strategies for Commercial Vision.”

