We at Hitachi Chemical Advanced Therapeutics Solutions, LLC are excited to announce that construction on additional PCT GMP manufacturing infrastructure and cleanroom capacity has begun at our newly-leased 49,700 square foot facility in Allendale, New Jersey (“75 Commerce”) in addition to upgrades to our existing facility at 4 Pearl Court, Allendale (“4 Pearl Court”). The upgrades will allow us to accommodate increasing demand for manufacturing and development services while seamlessly supporting and providing for both clinical and commercial applications.
Harmonized Global Service Platform
Advancing Commercialization
Expansion Planned in 2018
The 75 Commerce facility will house Grade B/ISO 7 controlled environment rooms, our Center for Innovation and Engineering, expanded and state-of-the-art manufacturing development laboratories, quality control and microbiology laboratories, warehousing, executive offices and convening space. As part of our expansion phase, 1,200 square feet of controlled environment rooms, 3,700 square feet of classified support areas, and additional support areas will be constructed, with facility validation completed by the end of 2018.
The existing 30,000 square foot manufacturing facility at 4 Pearl Court, housing six clean rooms, is now being renovated for improved process flow and commercial infrastructure with facility validation by the end of Q2 2018.
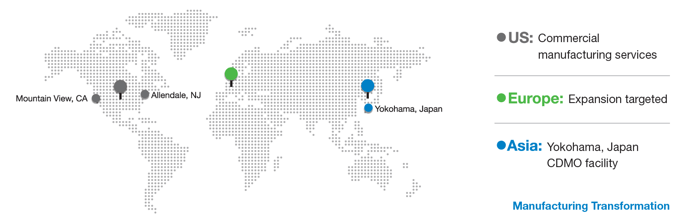
Now, through a total 145,000 sq ft footprint located between New Jersey, Japan (Yokohama), and California (Mountain View), clients can access the same Global PCT Service Platform for business, quality and information systems, manufacturing operations, and technology transfer protocols, ensuring a seamless experience for our clients and accelerating their global expansion plans through our global commercial manufacturing enterprise.
Thank you to our clients and prospective clients for your continued faith in HCATS as your provider and partner of choice for cell therapy manufacturing and development support.

*This page may include mention of our past company names as it reflects content distributed in the past. The former companies Hitachi Chemical Advanced Therapeutics Solutions (HCATS, fomerly PCT or PCT Cell Therapy Services) and apceth Biopharma GmbH have been renamed Minaris Regenerative Medicine.

